The magical effect of CAR-T therapy on brain cancer
Lead
On December 29th, the top medical journal New England Journal of Medicine published a clinical trial that resulted in an intracranial and intraventricular perfusion of CAR-T cells in an advanced glioma patient within weeks. The anti-tumor immune response in the patient was successfully achieved, and the tumor in the brain and the notochord was significantly atrophied, and there was no obvious adverse reaction. It has an extraordinary revelation for CAR-T therapy for other malignant tumors, especially solid tumors. It is another exciting news for the battle of immunotherapy against malignant tumors!
background
Glioblastoma: The most common primary malignant brain tumor with a high mortality rate. In the United States alone, more than 20,000 people are diagnosed with glioblastoma each year.
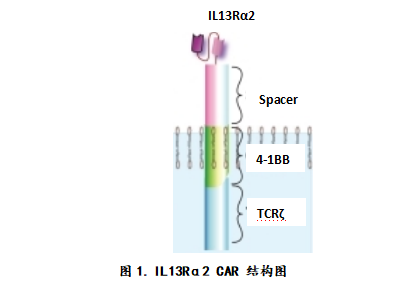
Richard Grady's story Interleukin-13 receptor alpha chain 2 (IL13Rα2) is a tumor-associated antigen of glioblastoma, and the high level of expression of this protein in tumors is closely related to the low survival rate of glioblastoma patients. The structure of IL13Rα2 is shown in Figure 1.
Richard Grady, a glioblastoma patient, like other cancer patients, has undergone routine treatments such as surgery, chemotherapy, and radiation therapy, but unfortunately, his tumors have recurred unexpectedly.

As in conventional CAR-T therapy, in the experiment, doctors extracted and isolated T cells from peripheral blood cells of Richard Grady, cultured and expanded them in vitro, and then genetically modified the expanded T cells to express targets. Chimeric antigen receptor (CAR) to IL13Rα2. After successful preparation of autologous CAR-T cells targeting IL13Rα2. Richard Grady has undergone the following treatment process.
Richard Grady's treatment process
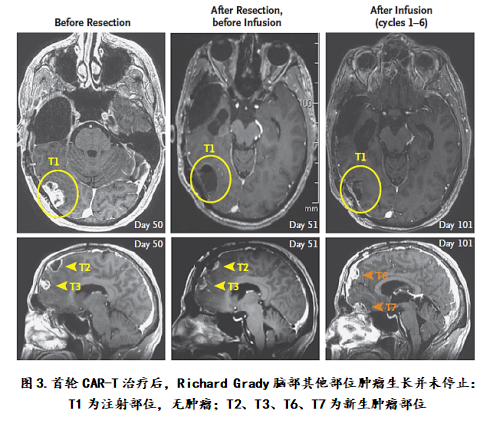
Stage 1: Autologous CAR-T cells targeting IL13Rα2 enter the intracranial 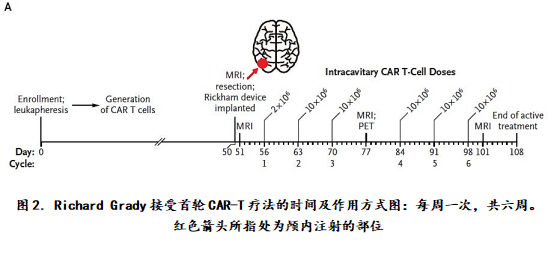 Therapeutic effect: After the end of the treatment, the tumor does not grow at the injection site, but there is a very frustrating situation, that is, the tumor remaining after surgery does not stop growing, and new tumor foci still appear, the tumor has even Spread to the notochord, it seems that the experiment has inevitably gone to the end of failure. Treatment: The doctors surgically resected three large tumors in the brain of Richard Grady, and then used the microtubules to intracranically prepare the prepared CAR-T cells for a total of six weeks of treatment at the site of the tumor. Treatment.
Therapeutic effect: After the end of the treatment, the tumor does not grow at the injection site, but there is a very frustrating situation, that is, the tumor remaining after surgery does not stop growing, and new tumor foci still appear, the tumor has even Spread to the notochord, it seems that the experiment has inevitably gone to the end of failure. Treatment: The doctors surgically resected three large tumors in the brain of Richard Grady, and then used the microtubules to intracranically prepare the prepared CAR-T cells for a total of six weeks of treatment at the site of the tumor. Treatment.
The researchers didn't give up on this, and they turned to another bold attempt to use another microtubule to inject CAR-T cells into the area of ​​cerebrospinal fluid produced by Richard Grady's brain . Because they believe that the previously limited efficacy is most likely caused by the inability of CAR-T to reach the site where tumor cells actually work.
The second stage: CAR-T cell perfusion to the site of cerebrospinal fluid production "The new treatment method is to use the fluidity of cerebrospinal fluid to bring T cells to different sites, and the movement route of T cells is consistent with the transfer route of tumor cells. Said Badie, director of neurosurgery at the City of Hope, who is responsible for the clinical trial.
 After three treatments, Richard Grady's tumor immediately violently atrophied and all the tumors disappeared! Richard Grady has been able to switch to other conventional therapies and has returned to work. Although he has been developing new tumors at different sites in Richard Grady's brain and notochord, he is also undergoing radiation therapy, but his response to immunotherapy has lasted for more than 7 months, for whom he survived at least There is still a year and a half, which is a very surprising result for the usual case of survival only a few weeks after the start of treatment. "The miracle is here!
After three treatments, Richard Grady's tumor immediately violently atrophied and all the tumors disappeared! Richard Grady has been able to switch to other conventional therapies and has returned to work. Although he has been developing new tumors at different sites in Richard Grady's brain and notochord, he is also undergoing radiation therapy, but his response to immunotherapy has lasted for more than 7 months, for whom he survived at least There is still a year and a half, which is a very surprising result for the usual case of survival only a few weeks after the start of treatment. "The miracle is here!
In addition, according to doctors, the side effects of Richard Grady's acceptance of the CAR-T therapy are very controllable. Some of the symptoms, including headaches, fatigue, and muscle aches, are said to be caused by other conventional therapies.
Follow-up progress
Finally, Badie revealed that nine patients have received their CAR-T immunotherapy, and three of them have adopted this new injection method. Only two of the nine patients reportedly had no response. This strongly suggests that CAR-T immunotherapy has a huge room for development in the treatment of recurrent solid tumors.
significance
Infusion of CAR-T cells into the cerebrospinal fluid site is the highlight and pioneer of this clinical trial. The new CAR-T treatment method accepted by Richard Grady is very promising for many solid tumor types such as lung cancer and breast cancer. The outlook is extremely broad. Because the efficacy of CAR-T immunotherapy is still very limited for solid tumors, the success of the new clinical application of Badie is of great significance for the CAR-T therapy of solid tumors.
References :
Christine E. Brown, Ph.D., Darya Alizadeh, Ph.D., et all. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N Engl J Med 2016; 375:2561-2569
Source: Medicalxpress: Cells dripped into the brain help man fight a deadly cancer
On December 29th, the top medical journal New England Journal of Medicine published a clinical trial that resulted in an intracranial and intraventricular perfusion of CAR-T cells in an advanced glioma patient within weeks. The anti-tumor immune response in the patient was successfully achieved, and the tumor in the brain and the notochord was significantly atrophied, and there was no obvious adverse reaction. It has an extraordinary revelation for CAR-T therapy for other malignant tumors, especially solid tumors. It is another exciting news for the battle of immunotherapy against malignant tumors!
background
Glioblastoma: The most common primary malignant brain tumor with a high mortality rate. In the United States alone, more than 20,000 people are diagnosed with glioblastoma each year.

Richard Grady's story Interleukin-13 receptor alpha chain 2 (IL13Rα2) is a tumor-associated antigen of glioblastoma, and the high level of expression of this protein in tumors is closely related to the low survival rate of glioblastoma patients. The structure of IL13Rα2 is shown in Figure 1.
Richard Grady, a glioblastoma patient, like other cancer patients, has undergone routine treatments such as surgery, chemotherapy, and radiation therapy, but unfortunately, his tumors have recurred unexpectedly.

Richard Grady
Richard Grady chose to register at the Hope City Cancer Center and become a cancer patient undergoing clinical trials. He is receiving a new form of cancer treatment, tumor immunotherapy, which is currently the most promising cure for cancer. The clinical trial was conducted by researchers at the Hope City Cancer Center in California and the Ben Towne Children's Cancer Research Center in Seattle. As in conventional CAR-T therapy, in the experiment, doctors extracted and isolated T cells from peripheral blood cells of Richard Grady, cultured and expanded them in vitro, and then genetically modified the expanded T cells to express targets. Chimeric antigen receptor (CAR) to IL13Rα2. After successful preparation of autologous CAR-T cells targeting IL13Rα2. Richard Grady has undergone the following treatment process.
Richard Grady's treatment process
Stage 1: Autologous CAR-T cells targeting IL13Rα2 enter the intracranial


The researchers didn't give up on this, and they turned to another bold attempt to use another microtubule to inject CAR-T cells into the area of ​​cerebrospinal fluid produced by Richard Grady's brain . Because they believe that the previously limited efficacy is most likely caused by the inability of CAR-T to reach the site where tumor cells actually work.
The second stage: CAR-T cell perfusion to the site of cerebrospinal fluid production "The new treatment method is to use the fluidity of cerebrospinal fluid to bring T cells to different sites, and the movement route of T cells is consistent with the transfer route of tumor cells. Said Badie, director of neurosurgery at the City of Hope, who is responsible for the clinical trial.

In addition, according to doctors, the side effects of Richard Grady's acceptance of the CAR-T therapy are very controllable. Some of the symptoms, including headaches, fatigue, and muscle aches, are said to be caused by other conventional therapies.

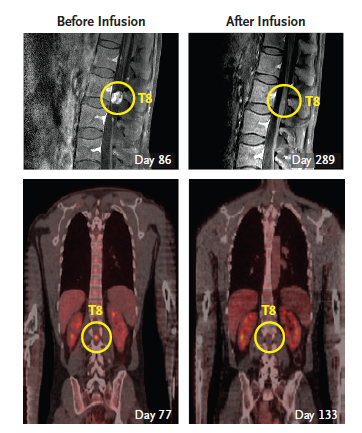
Figure 5. After the second round of CAR-T therapy , Richard Grady has a sharp atrophy of the tumor in the notochord
Follow-up progress
Finally, Badie revealed that nine patients have received their CAR-T immunotherapy, and three of them have adopted this new injection method. Only two of the nine patients reportedly had no response. This strongly suggests that CAR-T immunotherapy has a huge room for development in the treatment of recurrent solid tumors.
significance
Infusion of CAR-T cells into the cerebrospinal fluid site is the highlight and pioneer of this clinical trial. The new CAR-T treatment method accepted by Richard Grady is very promising for many solid tumor types such as lung cancer and breast cancer. The outlook is extremely broad. Because the efficacy of CAR-T immunotherapy is still very limited for solid tumors, the success of the new clinical application of Badie is of great significance for the CAR-T therapy of solid tumors.
References :
Christine E. Brown, Ph.D., Darya Alizadeh, Ph.D., et all. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N Engl J Med 2016; 375:2561-2569
Source: Medicalxpress: Cells dripped into the brain help man fight a deadly cancer
Hair Growth Meostherapy,Mesotherapy Treatment Injectable,Mesotherapy Scalpt Injection,Mesotherapy Cocktail Hair
Jiangsu Tiera Biotechnology Co., Ltd , https://www.tierabio.com