2017 new drug application volume will exceed 200
Medical Network December 22, China Medical Industry Information Center CPM database shows that as of December 20, 2017, the number of Class 1 chemical drugs undertaken by CDE reached 199, an increase of 42% over 2016. It is true that China’s innovative drug research and development is described as “explosive growthâ€.
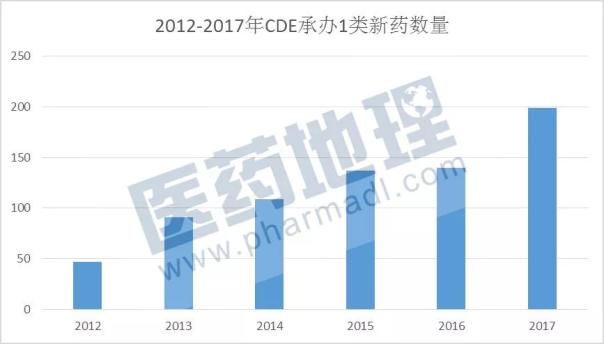
Source: China Pharmaceutical Industry Information Center CPM
From the data of the six years from 2012 to 2017, Hengrui is undoubtedly the pioneer of innovative drug research and development, and applied for 1 class of chemical drugs (excluding raw materials) to reach 17, Zhengda Tianqing and Guangdong Dongyang Sun each have 14 These three companies are in the first echelon of new drug research and development.
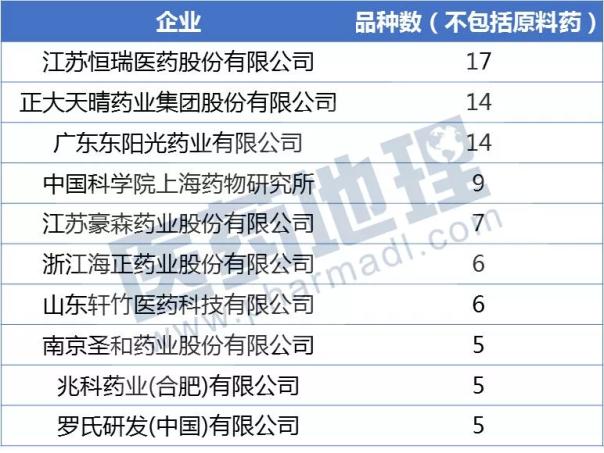
Source: China Pharmaceutical Industry Information Center CPM
In addition, we use the CPM database to analyze the number of new chemical products approved for marketing from 2012 to 2017 from the perspective of “the first batch of products approved for listingâ€. The new varieties here refer to drugs that have never been marketed in China (general purpose). Name + dosage form). After the three consecutive years of decline in the number of new varieties approved for listing, with the gradual resolution of the backlog of review tasks, 48 ​​new varieties were approved for listing in 2017. Speed ​​up the listing of new products, in order to better meet the clinical needs of our patients.
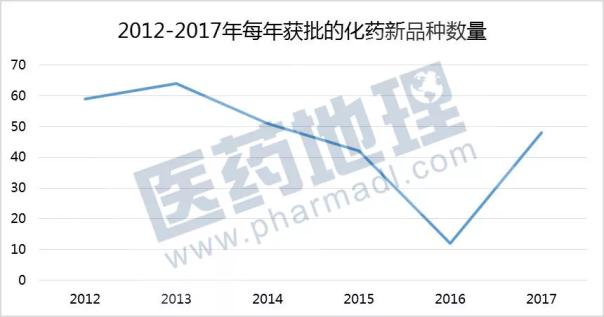
Source: China Pharmaceutical Industry Information Center CPM
The class of drugs specific to the declared 2017, in accordance with the current field of pharmaceutical treatment can be confirmed, the number of anti-tumor drugs topped, nervous system drugs, and anti-infectives ranked second and third. When the anti-cancer drugs are listed five years later, doctor education will determine the success or failure of the enterprise market strategy.
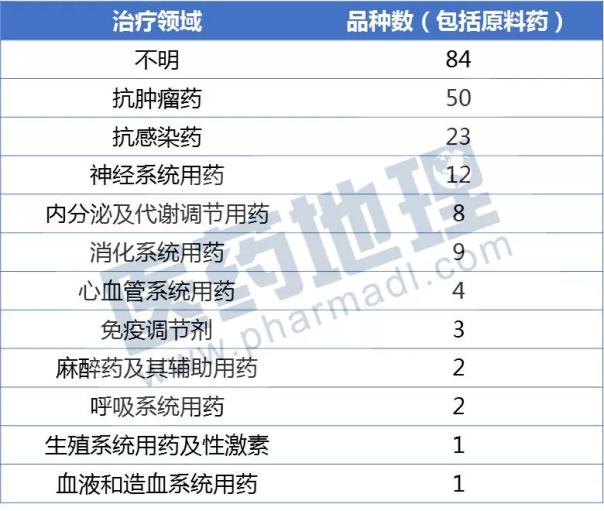
Source: China Pharmaceutical Industry Information Center CPM
From the perspective of the reporting company, Zhengda Tianqing became the big winner of the 2017 new drug registration application. Among the top ten, in addition to several companies that are familiar to everyone, there is also a relatively mysterious company - Hangzhou Ruomai Pharmaceutical Technology Co., Ltd. At present, the company has two products, RMX1001 capsules and RMX1002 tablets, which have been approved for clinical trials. The RMX1002 tablets have been approved and took 216 days.
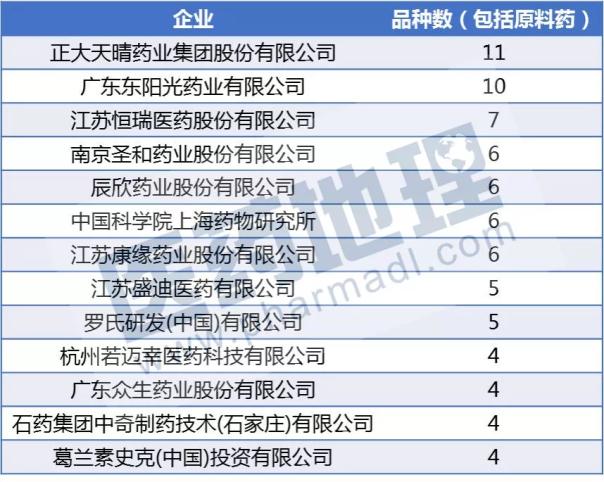
Source: China Pharmaceutical Industry Information Center CPM
Judging from the recent outbreak of the “medical insurance deficitâ€, the market prospects for innovative drugs may not be optimistic in the future. The medical reform hopes to find a balance between meeting clinical needs and coordinating medical insurance funds. In the next 5 to 10 years, after a large number of innovative drugs with similar efficacy will be listed, they will most likely face price stifling, which is exactly the same as the bidding dilemma faced by generic drugs today.
Central Gas Supply System
The
medical central gas supply system plays a significant role in modern hosptial.
The appropriate design and setup of medcal gas equipment are essential for the
hospitals to ensure medical quality, as well as to save the lives and ease the
pain of the gas station,Bed Head Unit,gas terminal, electrical system,nusre
call system, medical pipelines and auxilliary equipment system.
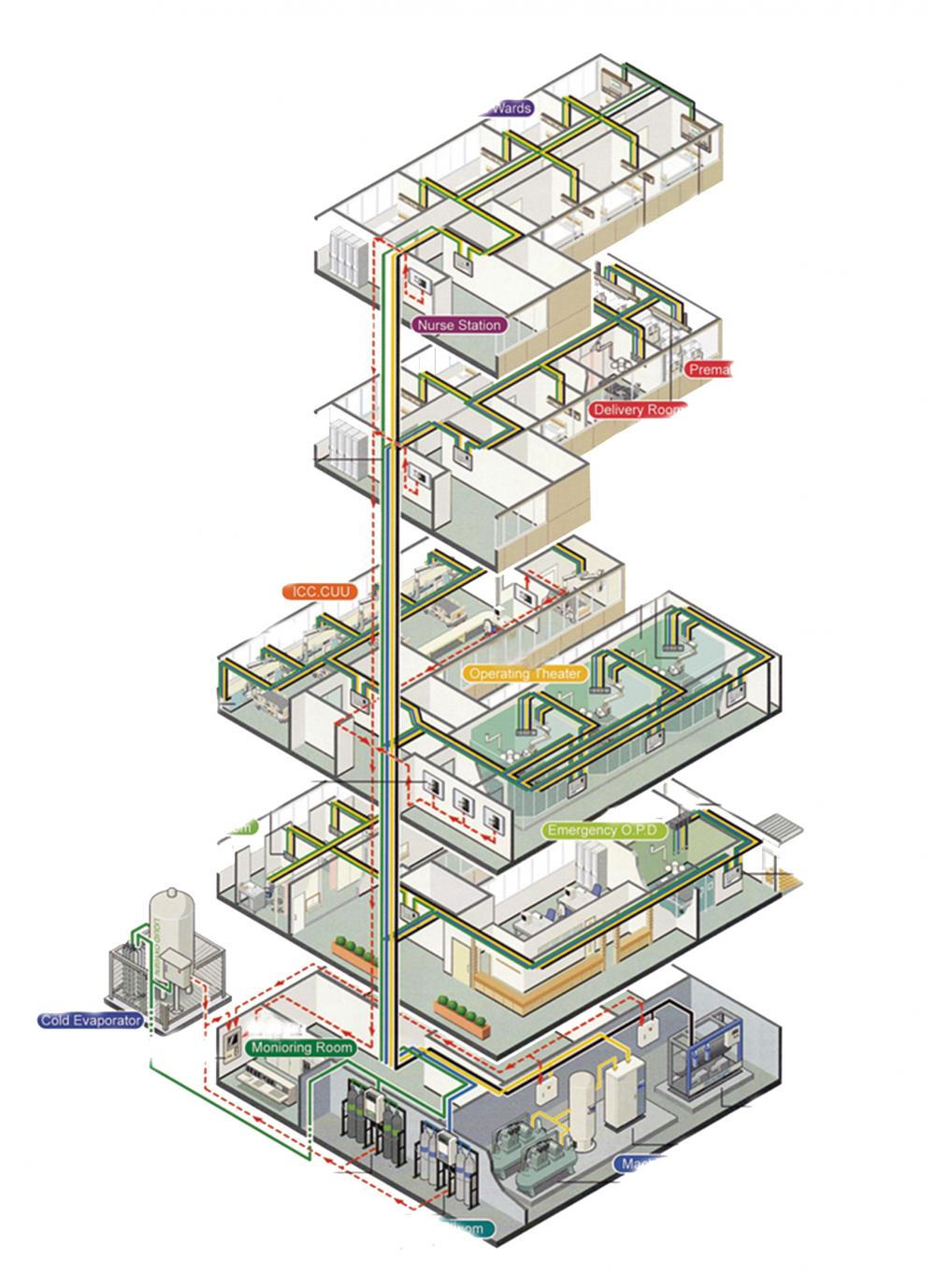
Central Gas Supply System,Hospital Central Gas Supply System,Hospital Gas Supply System ,Medical Gas Supply System
Hunan Eter Medical Co., Ltd. , https://www.eter-tech.com