Survival data is excellent, leukemia therapy publishes long-term follow-up results
Survival data is excellent, leukemia therapy publishes long-term follow-up results
December 06, 2017 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Biopharmaceutical company BioLineRx has announced a positive overall survival rate data for its long-term follow-up in a phase 2 clinical trial of the new drug BL-8040 for the treatment of relapsed or refractory (r/r) acute myelogenous leukemia (AML). The results showed that the combination of BL-8040 and HiDAC significantly improved overall patient survival compared to historical data from high-dose Ara-C (HiDAC) monotherapy.

Acute myeloid leukemia (AML) is an acute disease that begins in the bone marrow and has a large number of myeloblasts in the patient's bone marrow and blood. If left untreated, this leukemia can develop rapidly and can be fatal within a few months. AML originates in the bone marrow, but in most cases it quickly enters the bloodstream. Sometimes it spreads to other parts of the body, including lymph nodes, liver, spleen, central nervous system (brain and spinal cord) and testicles.
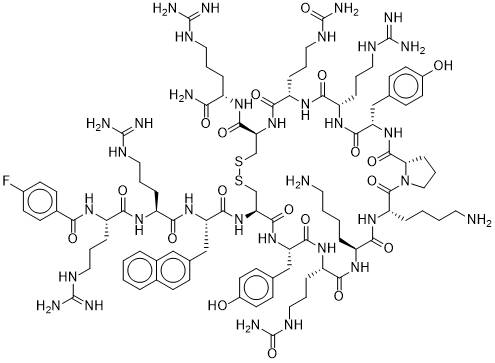
â–²Structure of BL-8040 (Source: medkoo)
BL-8040 is a circular short peptide which is a high affinity antagonist of the G protein coupled receptor CXCR4. CXCR4 is a chemokine receptor that is directly involved in tumor progression, angiogenesis, cell migration and survival. CXCR4 is overexpressed in more than 70% of human cancers, and its expression is often associated with disease severity. In many clinical and preclinical studies, BL-8040 shows strong mobilization of cancer cells and immune cells from bone marrow, making cancer cells sensitive to chemical and biological based anticancer therapies, and by inducing cell death (apoptosis) And mobilize immune cells to directly fight cancer. In addition, BL-8040 demonstrates powerful stem cell mobilization properties that mobilize a variety of cells, including colony forming cells, T, B, and NK cells.
Phase 2a studies evaluated the efficacy of BL-8040 as a single agent and in combination with HiDAC for the treatment of r/rAML. Most of the patients who participated in the study had undergone a number of other treatments, including patients who had relapsed after allogeneic stem cell transplantation, and patients with secondary AML, which represent a patient group that is difficult to treat and has a poor prognosis. The study enrolled 42 patients and the top-line results of all studies were published in March 2016. Compared with the historical data of HiDAC monotherapy (20% overall response rate), the overall response rate of patients receiving 38 mg/kg and above doses of BL-8040 (39 patients) increased to 38%.
â–²BioLineRx R&D pipeline (Source: BioLineRx official website)
The long-term survival results of the Phase 2a study of this report were derived from the extended phase of previous studies, including long-term follow-up of 16 of the 42 enrolled patients. Participants were treated with BL-8040 as monotherapy for two days, and then BL-8040 and HiDAC were combined for 5 days. The mean follow-up time was 338 days (29-853 days). The results showed that BL-8040 combined with HiDAC treatment was safe and well tolerated in patients with a response rate of 38% (6/16). The median overall survival of patients receiving combination therapy was 11.1 months (distribution interval 1-28 months) compared to the total survival of 6.1 months of historical data from patients receiving only HiDAC alone, based on this data. The one-year survival rate was 37.5% and the two-year survival rate was 28.5%. In addition, the subgroup of patients responding to treatment showed an extended overall survival time, measuring a survival rate of 60% for one and two years. Because only two of these patients relapsed, the median median overall survival of the group was temporarily uncalculated.

â–² Mr. Philip Serlin, CEO of BioLineRx (Source: BioLineRx official website)
Mr. Philip Serlin, CEO of BioLineRx, commented: "We are very pleased with the long-term follow-up of this study, which continues to demonstrate the robust anti-leukemia activity of BL-8040 and shows historical data compared to HiDAC Not only does it improve the response rate, but it also improves overall survival, and we will continue to focus on the long-term overall survival of patients in this study. We are also continuing two other important AML studies, including Phase 2a studies to maintain AML ( Part of the collaboration with Genentech) and our Phase 2b study to consolidate AML. The positive survival and remission rates we see in the r/rAML study, and the future data we expect in other studies in this disease area, will It is possible to make BL-8040 a key drug in the AML field."
We congratulate BioLineRx for the positive results and look forward to more new drugs to alleviate the pain of AML patients.
Reference materials:
[1] BioLineRx Reports Overall Survival Results From Long-Term Follow-Up of Phase IIa Trial in r/r AML
[2] BioLineRx official website
Press Lock Wire Seals are combined by a piece of wire and a plastic locking body. The locking body has some anchors to provide locking mechanism. So they are also called anchor seals. The anchor seal body is always made with polycarbonate material.
The anchor seals are originally designed as a replacement for lead seals. The application of the anchor seals include: Hospital Equipment, EMS Equipment, ATM Cassettes, Zipper Bags, Coin Boxes, Storage Units, Fibre Drums etc.
Press Lock Wire Seals,Press Lock Meter Seals,Press Lock Wire Seal,Meter Seals In Press Type
Wenzhou Haoshi Light Industrial Products Co., LTD , https://www.economicseals.com